The oxidation of sodium sulfide using transition metel oxides
THE OXIDATION OF SODIUM SULFIDE USING TRANSITION METAL OXIDES DEPOSITED ON THE POLYMER MATRIX
Смотрите также [PDF формат на английском языке]
Kazan is a large center of the Russian chemical industry.Production of polysulfide rubbers and hermetics occupy a special place in Kazan. In 2000 production of polysulfide hermetics for double-glazed windows was organized.
Thiocol production is extremely adverse in the ecological relation. It is well known that the odors from thiocol production are obnoxious. Many methods for eliminating Na2S have been reported due to industrial need. There will be some problems, however, if these methods are applied to wastewater treatment. The most interesting method of sodium sulfide detoxification is the oxidation of the toxic sulfur compounds in the waste by the use of atmospheric oxygen. In the absence of catalysts, this process is performed at temperatures of 90-110°C and pressures of 0.3-0.5 MPa. The use of catalysts can give a significant acceleration of the oxidation process, so that it can be performed at 40-50°C. Homogeneous catalysts, including transition metal oxides can dissolve in alkaline solution. Heterogeneous catalysts were synthesized by introducing transition metal oxides into the polymer matrix. The heterogeneous catalyst has a high level of chemical stability, mechanical strength, and stable catalytic activity.
In this paper is proposed the catalytic efficiency of transition metal oxides deposited on the polymer matrix in the sodium sulfide oxidation and investigation of kinetic parameters in presence of copper and manganese oxides catalyst.
The effect of transition metal oxides deposited on the polymer matrix in the sodium sulfide oxidation is given in Figure 1.
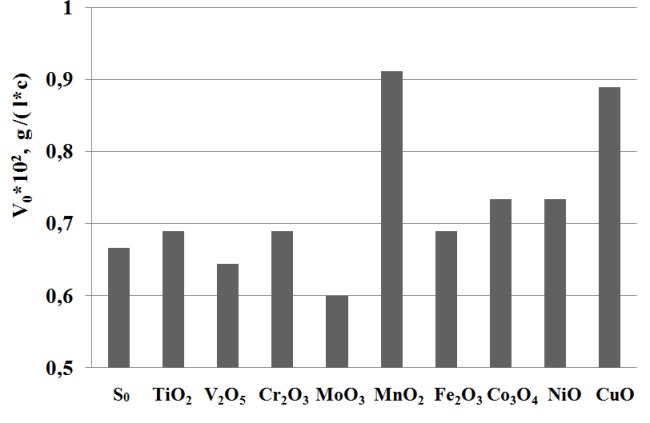
Fig. 1 The effect of transition metal oxides deposited on the polymer matrix in the sodium sulfide oxidation.
It is apparent from the Figure that copper and manganese oxides show maximum activity in the sodium sulfide oxidation, in this case intial rate of reaction is, respectively, about 1,4 and 1,35 times higher than intial rate of no catalyst. Oxides of NiO, Co3O4, Cr2O3, TiO2 — show insignificant activity, a part from the tested oxides: V2O5, Fe2O3 — don’t influence rate of reaction, and catalysts based on the MoO3 oxide- even inhibit sodium sulfide oxidation.
Catalytic activity of mixed compositions, which were synthesized by different concentration of copper and manganese oxides shows that CuO-5/MnO2-15 possesses highest activity for sodium sulfide oxidation (Figure 2).
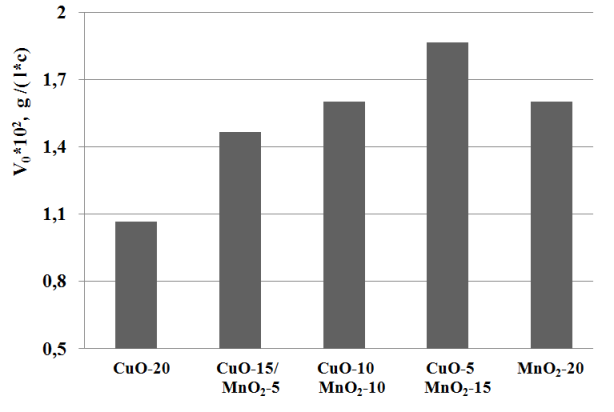
Fig. 2 The effect of mixed compositions by different concentration of copper and manganese oxides in the sodium sulfide oxidation.
Influence of the heterogeneous catalyst amount on the rate of reaction shows that increasing catalyst amount to 5,0 g leads to increase the rate of sodium sulfide oxidation. The further increases in catalyst amount don’t influence rate of reaction (Figure 3).
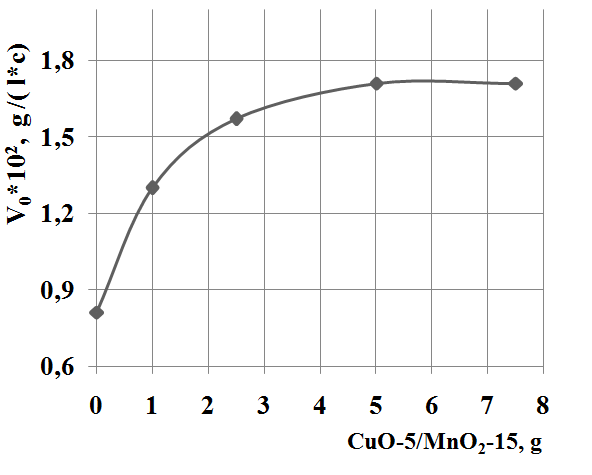 Fig. 3 Influence of the heterogeneous catalyst amount on the rate of sodium sulfide oxidation.
Fig. 3 Influence of the heterogeneous catalyst amount on the rate of sodium sulfide oxidation.
Influence of temperature on the rate of reaction shows that the maximum rate of sodium sulfide oxidation is observed at temperature 60°С, above and below 60°С rate of reaction is decreased (Figure 4).
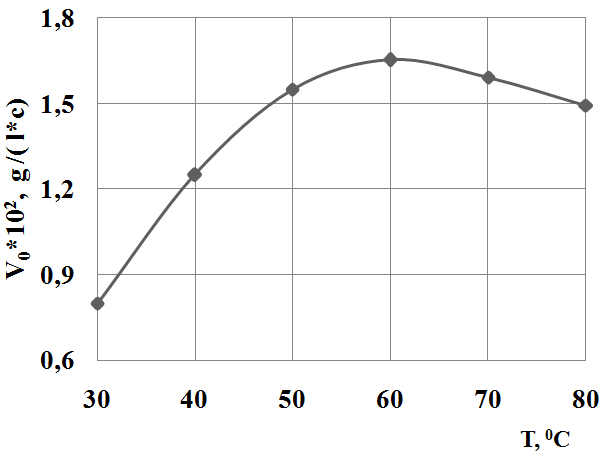
Fig. 4 Influence of temperature on the rate of sodium sulfide oxidation.
Kinetic methods show that all reactions are first order with respect to the [O2] and zero — to the concentration of sulfur compounds (Figure 5).
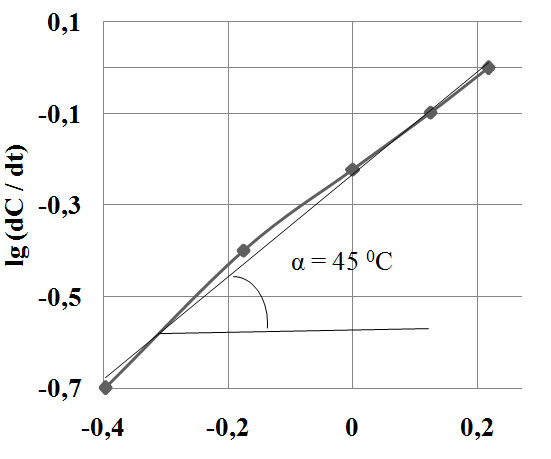
Fig. 5 Logarithmic dependence of rate of sodium sulfide oxidation on concentration О2.